Haryana’s health minister Anil Vij announced that he tested positive for the Covid-19, leading many to draw connections with his participation in the trials of one of the vaccine candidates. The immediate media coverage and the social media conversations that followed conflated Vij’s diagnosis with the trial in a way suggested that the inoculation of Covaxin from Bharat Biotech may be ineffective. In reality, it is not known whether the minister was given a placebo or the actual inoculation. Even if he did get a shot of Covaxin, it is not meant to be protective until he gets at least two doses to build the necessary protection against Covid-19.
Haryana’s health minister Anil Vij announced that he tested positive for the Covid-19, leading many to draw connections with his participation in the trials of one of the vaccine candidates. The immediate media coverage and the social media conversations that followed conflated Vij’s diagnosis with the trial in a way suggested that the inoculation of Covaxin from Bharat Biotech may be ineffective. In reality, it is not known whether the minister was given a placebo or the actual inoculation. Even if he did get a shot of Covaxin, it is not meant to be protective until he gets at least two doses to build the necessary protection against Covid-19.
Indeed this was disinformation at its worst. It became all the more sensational as it came couple of days after the Prime Minister Narendra Modi participated in the all party meeting via video conferencing to discuss the Covid-19 vaccination strategy in India. This had raised hopes of an early resolution of the pandemic. The meeting was also attended by the Union Defence Minister, Union Home Minister, Union Finance Minister, Union Minister for Rural Development, Union Minister for Social Justice and Empowerment and Union Minister for Parliamentary Affairs. The Prime Minister said that the government is developing a comprehensive vaccination strategy. He emphasized that the world is looking towards India for the development of a safe and affordable vaccine.
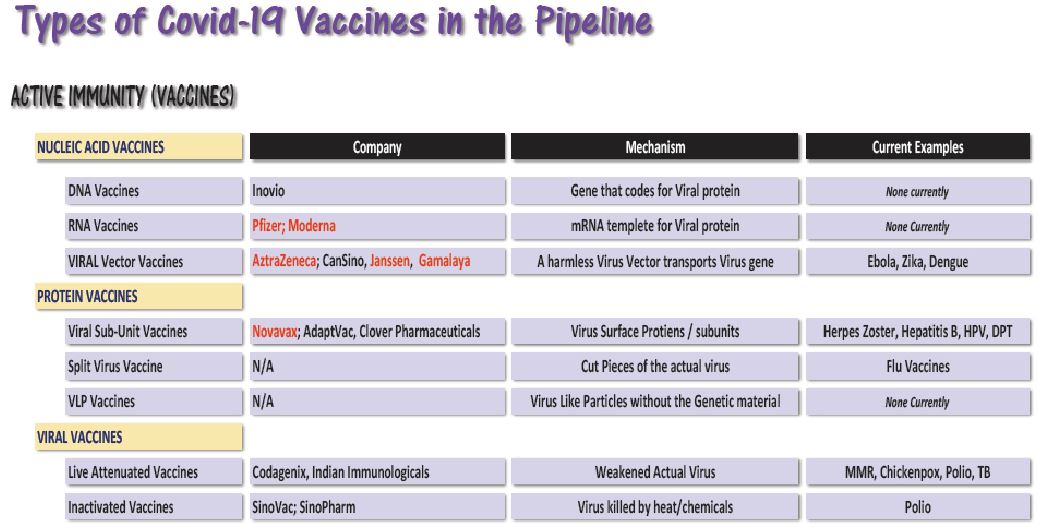
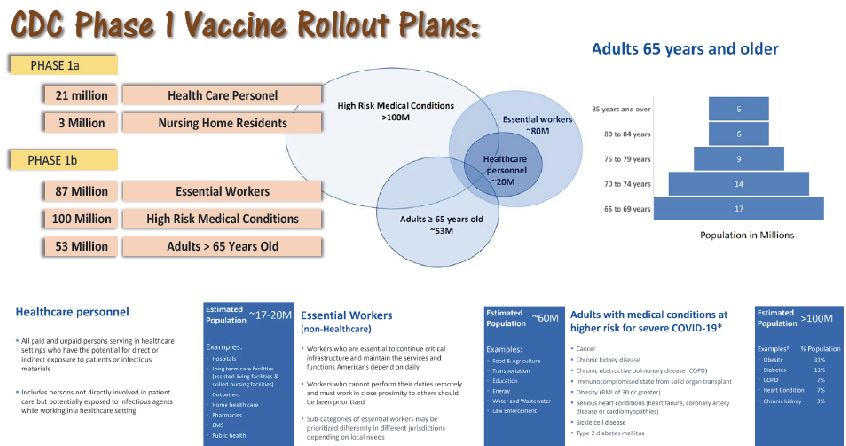
The Prime Minister shared his experience of visiting the vaccine manufacturing facilities in Ahmedabad, Pune, and Hyderabad, informing that about eight potential vaccines, currently in different stages of trial, will be manufactured in India, that includes the three indigenous vaccines. The Prime Minister said that it is expected that the vaccine will be available in the coming few weeks. The vaccination campaign will kick start in India as soon as the vaccine is approved by the scientists. The Central government is working in close coordination with the State governments to identify the priority groups for vaccination that will include Health Workers, Frontline workers and high-risk individuals viz the aged population cohort and people with comorbidities.
Preparedness for vaccine
In an effort to administer the vaccine in an efficient, smooth and transparent manner, database of healthcare and frontline workers, augmentation of cold chains and procurement of syringes and needles are in advanced stages of preparation.
However, collaboration with State Governments is crucial for additional cold chain equipment and other such logistical requirements. The digital platform for vaccine administration and distribution (Covid-19 Vaccine Information Network Co-WIN) is prepared and is being tested in collaboration with the State and District Level authorities and other stakeholders.
A National Expert Group that includes technical experts and officials of both the Central Government and State Governments, has been constituted to shoulder the responsibility of the campaign related to the vaccine.
The National Expert Group will take decisions collectively as per national and regional requirements. India’s strategy has been well received by the globe and the Prime Minister mentioned that Indians have fought this pandemic with indomitable will, noting that the restraint, courage, and strength of Indians has been incomparable and unprecedented during this entire battle.
He added that we not only helped our fellow Indians but also made every effort to save the citizens of other countries as well. Also, the scientific methodology adopted by India led to increased testing in India, which not only reduced the positivity rate but also reduced the covid mortality rate.
The Prime Minister cautioned against rumors that may be spread about vaccination, saying that it would be against both public interest and national interest. He called upon all the leaders to make the citizens of the country more aware, and prevent any such rumors from spreading.
India has already reportedly asked for 500 million doses of the Oxford University-AstraZeneca vaccine candidate, one billion from the US company Novavax and 100 million doses of the Sputnik V candidate from Russia’s Gamaleya Research Institute, according to the US-based Duke University Global Health Innovation Center. The ‘Launch and Scale Speedometer’ analysis, which is updated every two weeks, shows India has confirmed 1.6 billion doses of three vaccines as of November 30 while the US and the EU have purchased doses of six candidates.
According to the analysis, India is the top COVID-19 vaccine buyer followed by the European Union which has confirmed 1.58 billion doses and the US, the worst affected country so far, that has managed to purchase just over a billion doses.
 As India is the largest buyer of COVID-19 vaccines in the world with 1.6 billion doses, according to a global analysis, a number some scientists say could cover 800 million people, or 60 per cent of its population, and will be enough to develop ‘herd immunity’.
As India is the largest buyer of COVID-19 vaccines in the world with 1.6 billion doses, according to a global analysis, a number some scientists say could cover 800 million people, or 60 per cent of its population, and will be enough to develop ‘herd immunity’.
In November, Union Health Minister Harsh Vardhan had said 400-500 million doses of COVID-19 vaccines were estimated to be made available for 250 to 300 million (25 crore to 30 crore) people in India by July-August 2021.
Countries such as Bangladesh, Myanmar, Qatar, Bhutan, Switzerland, Bahrain, Austria and South Korea – have shown keen interest in partnering for vaccine development of Indian vaccines and use thereof. In an effort to administer the vaccine at the first available opportunity, database of healthcare and frontline workers, augmentation of cold chains and procurement of syringes, needles, etc. are in advanced stages of preparation. The vaccination supply chain is being enhanced and non-vaccine supplies are being escalated. Medical and nursing students and faculty will be involved in training and implementation of the vaccination programme. Every step is being steadily put in place to ensure that vaccines reach every location and person according to the prioritization principles.
 National Expert Group on Vaccine Administration for Covid-19 (NEGVAC) in consultation with State Governments and all relevant stakeholders have accelerated the implementation of vaccination of priority groups in first phase. The digital platform for vaccine administration and distribution is prepared and test runs underway in partnership with the State and District Level stakeholders. The Government has provided assistance of 900 crore under Covid Suraksha Mission to support Research & Development of Covid-19 vaccination.
National Expert Group on Vaccine Administration for Covid-19 (NEGVAC) in consultation with State Governments and all relevant stakeholders have accelerated the implementation of vaccination of priority groups in first phase. The digital platform for vaccine administration and distribution is prepared and test runs underway in partnership with the State and District Level stakeholders. The Government has provided assistance of 900 crore under Covid Suraksha Mission to support Research & Development of Covid-19 vaccination.
However, the news that Haryana’s Health Minister had been infected by Covid-19 even after administration of vaccine, should be taken with a pinch of salt. This is the case of inadequate information gaining traction on social media. Earlier, the coverage of the adverse effect in a volunteer participating in the Oxford-AstraZeneca vaccine trial in Chennai amplified allegations that were later disproved.
letters@tehelka.com